The Parameters Of Semen Analysis
Semen analysis is a complex laboratory test and detects many aspects of spermatozoa. The first step is the macroscopic evaluation. Immediately after ejaculation a semen sample is in the form of a gel. The spermatozoa at this point are immotile because they are “trapped” in this mesh. Over time, the sample liquefies and becomes like water. The normal liquefaction process can take from a few minutes to half an hour.
At the macroscopic evaluation of the sample volume and pH are also recorded as well as the presence of aggregations and agglutinations which are also seen in some samples. Aggregations and agglutinations refer to spermatozoa that are stuck to each other and as a result they cannot move towards the oocyte.
The microscopic evaluation describes many aspects of spermatozoa that include the concentration of spermatozoa, their motility and vitality as well as their morphology. Apart from spermatozoa other cell types, such as the round cells, that exist in the sample are also counted and differentiated.
A semen sample is considered “fertile” when
i) it has a sufficient number of spermatozoa,
ii) a significant percentage of the spermatozoa have a good motility, which means that they can reach the oocyte and
iii) there are spermatozoa with good morphology which indicates that the spermatozoa can travel through the cervical mucus in the female reproductive track and find the oocyte.
1. Counting Spermatozoa
The number of spermatozoa that exist in the semen sample is a very important parameter. In order to count the number of spermatozoa
i) the sample is first diluted
ii) then is “loaded” onto a plate called “Newbauer cytometer”.
iii) the spermatozoa are counted and after calculations the number of spermatozoa per ml is reported.
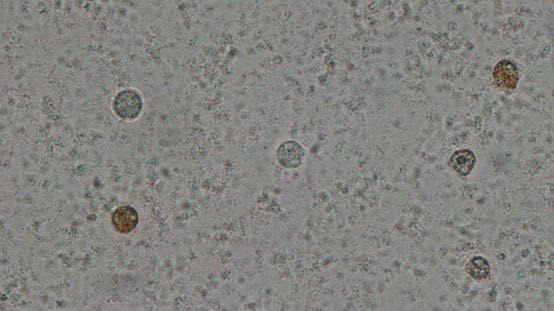
2. Round cells in semen
A semen sample contains spermatozoa as well as round cells. As shown in the following video some semen samples can contain a large number of round cells but by looking at the microscope there is no way to differentiate between the two cell types.
So these cells are
i) counted in millions per ml, the way is done for spermatozoa and
ii) are differentiated and classified into 2 categories: white blood cells and immature germ cells.
The differentiation is done by the peroxidase method, that stains the white blood cells brown whereas the immature germ cells remain white.
3. Sperm motility
The spermatozoon must reach the oocyte in order to fertilize it. It is therefore important to know the motility of spermatozoa in a semen sample.
1. Spermatozoa can have fast forward progressive motility
2. Spermatozoa can have slow forward progressive motility
3. Spermatozoa can have non-progressive motility
Sperm motility is evaluated exclusively in a phase contrast microscope, which magnifies the spermatozoa 400 times. It is important to count a sufficiently large number of spermatozoa in order for the count to be accurate. The motility result indicates the percentage of fast moving spermatozoa, the percentage with moderate (sluggish) motility, the spermatozoa that have non-progressive motility and those that are immotile.
Motility measurements are difficult to perform properly and are only carried out by an experienced observer.
To date, there are NO automated analyzers that can give reliable results and this is the reason why they are never used routinely. Automated sperm analyzers are only useful in research protocols, because they can accurately measure certain sperm characteristics. That is why, as the World Health Organization (WHO) concludes, sperm analyzers are not yet ready for use in diagnostic laboratories.
4. SPERM VITALITY
There are both live and dead spermatozoa in every semen sample. It is important to evaluate the vitality of spermatozoa in every sample, especially in samples with less than 40% progressive motility. This test is used as a check for the motility of spermatozoa, since the percentage of dead spermatozoa cannot exceed the percentage of immotile spermatozoa. On the contrary the percentage of viable can normally exceed the percentage of motile spermatozoa. Usually two methods are used for vitality evaluation, the eosin-nigrosin method and the hypoosmotic swelling.
Eosin- nigrosin method
The most common method for the vitality of spermatozoa is the eosin-nigrosin method. This method is based on the use of a dye that cannot cross the cell membrane, when a cell is alive. As a result all spermatozoa that are alive remain white while the dead ones stain red, as shown in the picture.

HYPO-OSMOTIC SWELLING
Another test for the evaluation of vitality is the hypo-osmotic swelling test. This test is based on the principle that a live cell, that therefore has a functional membrane, will swell in a hypotonic solution. In the case of spermatozoa the tail will swell as shown in the following video
5. SPERM MORPHOLOGY
The morphology of spermatozoa provides information on the size and shape of each spermatozoon. Morphology evaluates all parts of the spermatozoa i.e. the head, the neck and the tail as well as the cytoplasmic droplet that might exist. Studies have shown that spermatozoa with normal morphology can fertilize the oocyte. In order to evaluate the morphology, first the sample will be subjected to a specific treatment and then it will be stained with the “Papanikolaou” technique. Thus, the spermatozoa are colored and the abnormalities that appear in each sperm’s head, neck or tail can be accurately evaluated, as shown in the video that follows.
The sperm head may have abnormalities in size or shape. It may be smaller or larger than normal and its shape may differ from normal and may be elongated, pear-shaped, amorphous, etc. Of great importance is the evaluation of the acrosome, the area that has a light color on the tip of the spermatozoon’s head. The acrosome contains enzymes that will “pierce” the oocyte’s shell so that the spermatozoon can enter and fertilize it. In cases where the acrosome is absent or very small, the egg cannot be fertilized.
The midpiece is the area of the spermatozoon that contains the mitochondria. The midpiece can be “broken”, thinner or occasionally have a cytoplasmic residue. The tail of the sperm can be cut, double, wrapped etc.
The morphology assessment also includes the measurement of the teratozoospermia index (TZI), which shows how many abnormalities each abnormal spermatozoon has.
Morphology is an important parameter because it is the parameter that is usually stable between different semen analysis tests of the same individual – unlike the concentration and the motility. The morphology can also provide information about genetic abnormalities that alter the sperm and affect the formation of the fetus. Such conditions are globozoospermia and short tail syndrome and the only way to detect them is by accurately assessing the morphology of spermatozoa with the Papanicolaou staining.
Globozoospermia is a condition in which the head of the spermatozoa have the form of a perfect circle and lack the acrosomal area. In these cases natural conception cannot occur in the couple.
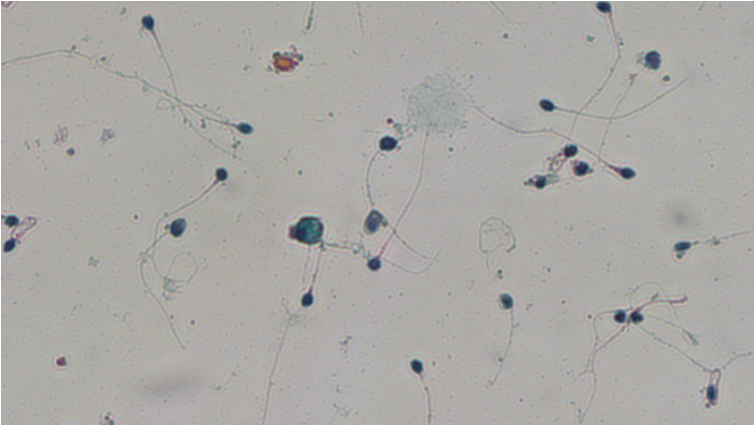
Short tail syndrome describes cases in which the spermatozoa do not have normal tails. In these samples the tails are very small, they look like they are cut and the motility of these sample is almost zero. As a result the spermatozoa cannot reach the oocyte and therefore natural conception cannot be achieved.
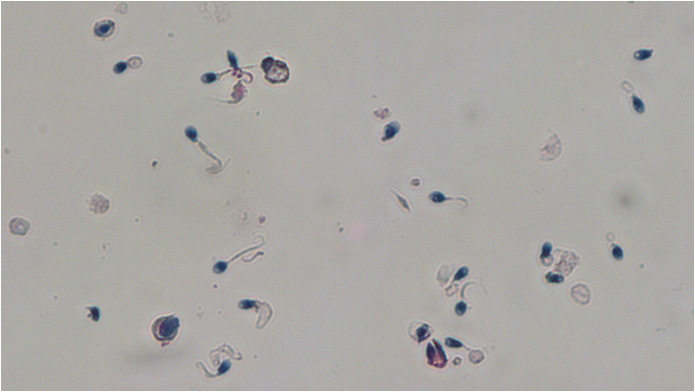

General Information
περισσότερα

FAQs
περισσότερα

Instructions Before The Semen Analysis
περισσότερα


